Advancing Treatments
Leveraging our unparalleled expertise in diseases of the immune system and CXCR4 biology, we aim to bring innovative treatments to people with rare diseases of the immune system.
Our Focus on CXCR4
CXCR4, or C-X-C receptor type 4, and its ligand, CXCL12, have been shown to play a key role in regulating the mobilization of white blood cells, including neutrophils, lymphocytes, and monocytes, from the bone marrow into peripheral circulation. Because inhibition of the CXCR4 receptor has been shown in the clinic to elevate levels of circulating white blood cells, we believe that therapies targeting the CXCR4/CXCL12 pathway hold the potential to benefit patients across a variety of diseases of the immune system.
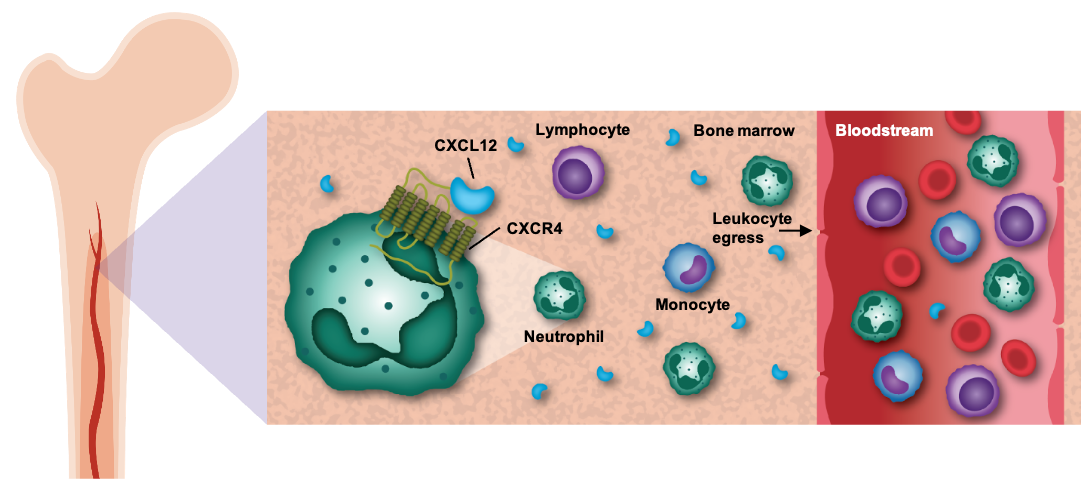
The CXCR4 pathway regulates the mobilization of white blood cells from the bone marrow to the peripheral blood
About Mavorixafor
Our deep understanding of the biology of the CXCR4 pathway has enabled us to successfully develop our first product, mavorixafor, in its first indication. In April 2024, we received FDA approval of mavorixafor, which is being marketed in the U.S. under the trade name XOLREMDI®, for use as an oral, once-daily therapy in patients 12 years of age and older with WHIM syndrome to increase the number of circulating mature neutrophils and lymphocytes. XOLREMDI is the first drug ever approved in the U.S. to treat WHIM syndrome.
Mavorixafor has received multiple special designations from global regulatory authorities in WHIM syndrome, including Breakthrough Therapy Designation, Fast Track Designation, and Rare Pediatric Designation in the United States, and orphan designation in both the United States and European Union.
Commercializing Mavorixafor in WHIM Syndrome
United States
We launched XOLREMDI in the U.S. in May 2024 and are currently promoting the drug through our X4 commercial team. We have deployed a dedicated field force of Medical Science Liaisons, Patient Diagnostic Liaisons, and a nurse educator in the United States to drive education and awareness of WHIM syndrome and its diagnosis.
European Union
The European Medicines Agency (EMA) is currently reviewing the Marketing Authorization Application (MAA) for mavorixafor in the treatment of WHIM syndrome. X4 has entered into a licensing and supply agreement with Norgine, a leading European specialist pharmaceutical company, under which Norgine will commercialize mavorixafor in the EU following any regulatory approvals.
Middle East / North Africa (MENA) Region
X4 and taiba rare, a company with specialty & orphan drug marketing, sales, and distribution expertise in the MENA region, have entered into an exclusive agreement for the distribution and commercialization of XOLREMDI in Saudi Arabia, United Arab Emirates, Qatar, Oman, Kuwait, Bahrain, and Egypt, following any approvals in the territories.
Advancing Mavorixafor in Chronic Neutropenic Disorders
- Chronic neutropenia is a rare blood condition defined by a decrease in blood neutrophil counts lasting more than three months; people with chronic neutropenia are at higher risk of developing infections and certain cancers and having a reduced quality of life.
- There has been little innovation for people with chronic neutropenia over the past 30 years; the only medicine approved to treat those living with severe chronic neutropenia is granulocyte colony-stimulating factor (G-CSF), an injectable therapy.
- Following successful completion of Phase 1b and Phase 2 clinical trials exploring the use of once-daily oral mavorixafor in the treatment of certain chronic neutropenic disorders, we have initiated a pivotal, global Phase 3 clinical trial.
- The Phase 3 4WARD trial (NCT06056297) aims to evaluate the efficacy, safety, and tolerability of oral once-daily mavorixafor (with or without G-CSF) in people with congenital or acquired primary autoimmune and idiopathic chronic neutropenia who are experiencing recurrent and/or serious infections. For more information, please visit 4WARDstudy.com.
